
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
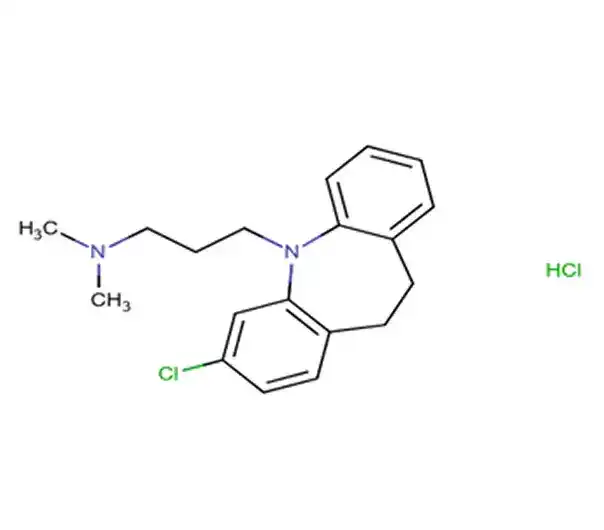
API
PN*
C57H83N3O39S
(1S,3R,5R,6S,8R,10R,11S,13R,15R,16S,18R,20R,21S,23R,25R,26S,28R,30R,31S,33R,35R,36R,37R,38R,39R,40R,41R,42R,43R,44R,45R,46R,47R,48R,49R)-5,10,15,20,25,30,35-heptakis(hydroxymethyl)-2,4,7,9,12,14,17,19,22,24,27,29,32,34-tetradecaoxaoctacyclo[31.2.2.2…]nonatetracontane-36,37,38,39,40,41,42,43,44,45,46,47,48,49-tetradecol; 4-hydroxy-2-methyl-1,1-dioxo-N-pyridin-2-yl-1λ⁶,2-benzothiazine-3-carboxamide
96684-39-8
1466.33 g/mol
NSAID
| Appearance | Off-white to pale yellow crystalline powder or granules |
|---|---|
| Solubility | Significantly increased aqueous solubility compared to free piroxicam (up to ~100x improvement) |
| Melting point | Broad or lower melting range due to complex formation (typically lower than pure piroxicam) |
| pH | Neutral to slightly alkaline in aqueous solution due to β-cyclodextrin |
Piroxicam Beta-Cyclodextrin is a pharmaceutical complex that combines piroxicam, a non-steroidal anti-inflammatory drug (NSAID), with beta-cyclodextrin, a cyclic oligosaccharide. This combination enhances solubility, dissolution rate, and gastrointestinal tolerability of piroxicam.
Inhibits cyclooxygenase (COX-1 and COX-2) enzymes, reducing the synthesis of prostaglandins involved in pain, inflammation, and fever.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Primarily administered orally, though enhanced solubility may allow for development of other dosage forms like injectables or topicals with further research.
β-Cyclodextrin is pharmacologically inert and acts solely as a carrier molecule to enhance drug solubility and stability.
Dosing is generally equivalent since the same amount of active drug is delivered, but improved bioavailability may allow for better efficacy at similar doses.
Salius Pharma supplies Piroxicam Beta Cyclodextrin as an Active Pharmaceutical Ingredient (API).
To order, contact Salius Pharma with details such as desired quantity, target market, and regulatory requirements like DMF or GMP certificates.
Salius Pharma exports to regions worldwide, including Asia, Europe, the Middle East, Africa, North America, and South America.
Looking to source Piroxicam Beta Cyclodextrin or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.