
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/EP
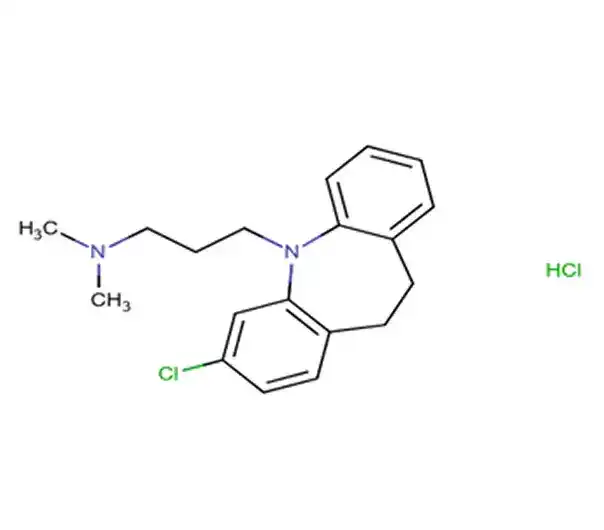
C24H31NO4 · HCl
1-[(3,4-diethoxyphenyl)methyl]-6,7-diethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride
69975-86-6
433.97 g/mol
Isoquinoline derivative
Anti-Spasmodic
| Appearance | Yellow to orange crystalline powder |
|---|---|
| Solubility | Freely soluble in water, methanol and ethanol |
| Melting point | 198–200°C |
| pH | 4.5–5.5 |
Drotaverine HCl is a smooth muscle relaxant belonging to the isoquinoline chemical class. It works by inhibiting phosphodiesterase-IV (PDE4), leading to increased levels of cyclic AMP (cAMP) in smooth muscle cells, which results in muscle relaxation. Unlike anticholinergic agents, drotaverine does not cause dry mouth or blurred vision, making it a safer option for relieving spasms.
Drotaverine HCl works by selectively inhibiting the enzyme phosphodiesterase-IV (PDE4) in smooth muscle cells, which leads to an increase in cyclic AMP (cAMP) levels. Elevated cAMP causes relaxation of smooth muscles by reducing calcium ion concentration inside the cells, thereby relieving spasms and cramps without affecting normal muscle tone. Unlike anticholinergic agents, drotaverine does not block acetylcholine receptors, so it avoids common side effects like dry mouth and blurred vision. This mechanism makes it effective for treating smooth muscle spasms in conditions such as abdominal pain, renal colic, and menstrual cramps.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It is widely used in gastroenterology, gynecology, and urology for relief of smooth muscle spasms such as abdominal colic, biliary colic, renal colic, and dysmenorrhea.
No anticholinergic side effects, fast spasm relief, strong safety profile, and compatibility with multiple dosage forms including tablets and injections.
Full regulatory package including CoA, DMF (where applicable), stability data, GMP certificates, CTD/eCTD support, and country-specific registration guidance.
Pharmaceutical-grade sealed HDPE/Fiber drums (usually 25 kg) with moisture-control liners suitable for long-distance shipment and varied climate zones.
Yes, Salius Pharma exports Doxofylline API to several African countries. As part of its global expansion strategy, Salius Pharma is actively engaged in supplying high-quality pharmaceutical ingredients to various regions, including Africa. The company has established a presence in countries such as Nigeria, Kenya, South Africa, Ethiopia, and Ghana, among others.
Store in tightly closed containers at controlled temperature, protected from humidity and direct light to prevent degradation or clumping.
Looking to source Drotaverine HCl or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.