
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP
C14H11Cl2NO4
2,2-Dichloro-N-(2-hydroxyethyl)-N-[(5-nitro-1,3-thiazol-2-yl)methyl]acetamide furan-2-carboxylate
3736-81-0
328.147
Imidamides
Anti-Diarrheal
| Appearance | Off-White Crystalline Solid |
|---|---|
| Solubility | Slightly soluble in Chloroform and Methanol |
| Melting point | 111-114 °C |
| pH | 4.5 – 6.5 |
Diloxanide furoate is used alone as a primary agent in the treatment of asymptomatic (cyst passers) intestinal amebiasis caused by Entamoeba histolytica. Diloxanide may also be used concurrently, or sequentially, with other agents such as the nitroimidazoles (e.g. metronidazole) in the treatment of invasive or extraintestinal forms of amebiasis.
Diloxanide furoate is an oral amoebicidal agent that works by being hydrolyzed in the intestine to its active form, diloxanide, which then targets the trophozoites of Entamoeba histolytica in the intestinal lumen. It disrupts the parasite’s metabolic processes, leading to their death and clearance from the gut. Since it acts locally in the intestines and is minimally absorbed into the bloodstream, it is effective mainly against non-invasive intestinal amoebiasis.
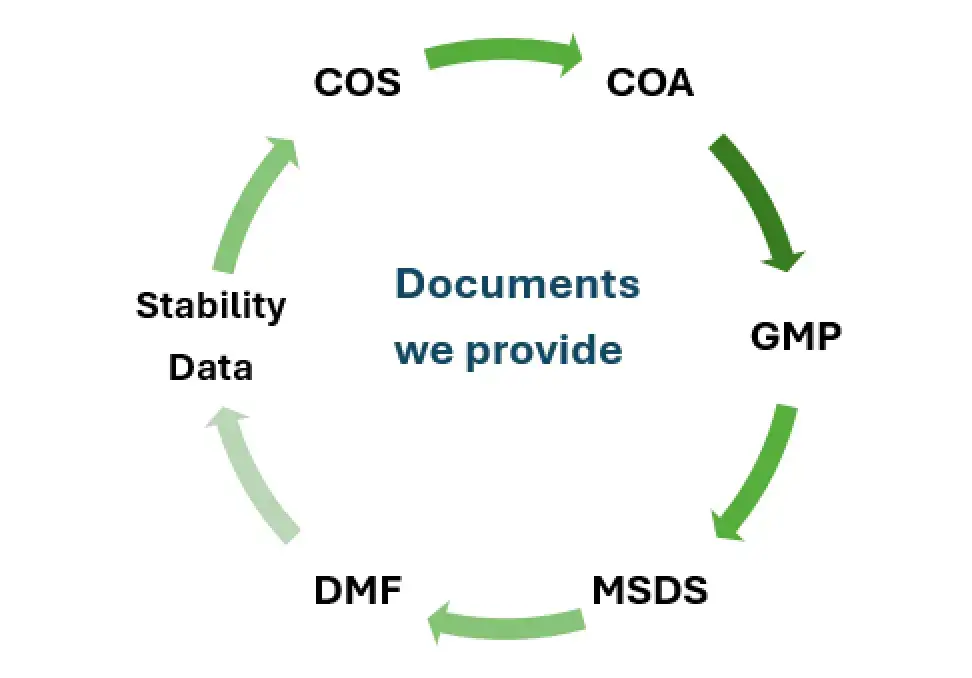
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Countries with higher prevalence of amoebiasis such as Africa, Latin America, Southeast Asia, and the Middle East drive strong market demand.
It effectively targets and eliminates intestinal amoebiasis carriers with minimal systemic absorption and fewer side effects, making it ideal for broad use.
Pharmacopeial compliance (BP/USP), tight impurity control, suitable particle size for tablet formulations, and validated stability data.
GMP certifications, CoA, MSDS, DMF/CTD support, and region-specific dossier assistance for smooth import approvals.
Lead time generally ranges 2–4 weeks depending on order size and documentation. MOQ is usually 25 kg, with flexibility for strategic partners and large-volume purchasers.
Store at controlled room temperature, protect from moisture and light, and ensure proper sealing to prevent degradation during long-distance shipping.
Looking to source Diloxanide Furoate or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.