
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
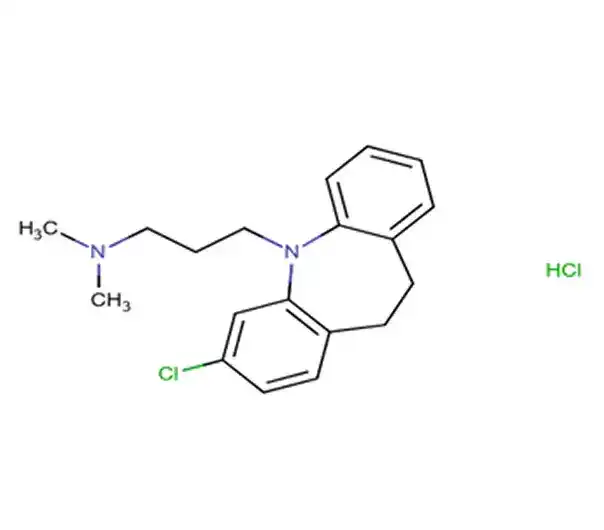
C18H26Cl2N2O2
Diethylazanium;2-[2-(2,6-dichloroanilino)phenyl]acetate
15307-79-6
373.32 g/mol
Dichlorobenzenes
Anti-Inflammatory Agents
| Appearance | White to slightly yellowish, crystalline powder. |
|---|---|
| Solubility | Practically insoluble in water, soluble in methanol and ethanol, slightly soluble in chloroform |
| Melting point | 134°C to 136°C |
| pH | 4.0 to 6.0 |
Diclofenac Diethyl ammonium acts by reducing inflammation and by extension reduces nociceptive pain and combats fever. It also increases the risk of developing a gastrointestinal ulcer by inhibiting the production of protective mucus in the stomach.
The enzymes cyclooxygenase-1 and -2, which produce prostaglandin (PG) G2, the building block of other PGs, are inhibited by diclofenac diethyl ammonium. The mechanism that unites all of Diclofenac Diethyl Ammonium's effects is the prevention of these molecules synthesis, which has a wide range of action in pain and inflammation.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Salius Pharma supplies Diclofenac Diethylammonium API in HDPE or fiber drums with double-layer polyethylene liners. Packaging complies with international transport and GMP standards and can be customized upon request.
The typical MOQ for Diclofenac Diethylammonium API is 25 kg. Larger volumes and flexible batch sizes can be arranged based on customer needs and destination requirements.
Salius Pharma provides complete technical documentation including DMFs, CoAs, and stability data. Support is available for country-specific regulatory submissions across markets such as Africa, LATAM, and CIS.
API shipments are accompanied by a commercial invoice, CoA, MSDS, packing list, Certificate of Origin, and, if required, a Free Sale Certificate. All documents are prepared to ensure seamless customs clearance and compliance.
Salius Pharma’s export facility handles large-scale API dispatches with strict quality checks and global logistics coordination. They ensure temperature-controlled shipping, export labeling, and real-time tracking support.
Yes, Salius Pharma exports Diclofenac Diethyl ammonium to key LATAM markets such as Mexico, Colombia, Peru, and Argentina. The company offers region-specific regulatory documentation, along with customized packaging, logistics support, and reliable supply chain solutions tailored to Latin American regulatory and market needs.
Salius Pharma’s export facility handles large-scale API dispatches with strict quality checks and global logistics coordination. They ensure temperature-controlled shipping, export labeling, and real-time tracking support.
Looking to source Diclofenac Diethyl ammonium or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.