
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
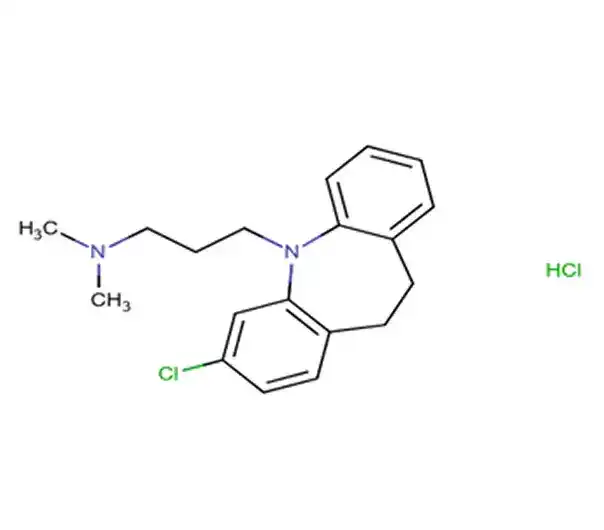
API
BP/USP
C19H19ClN2
13-chloro-2-(piperidin-4-ylidene)-4-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,12,14-hexaene
100643-71-8
310.821 g/mol
Benzocycloheptapyridines
Histamine Antagonists
| Appearance | White to slightly yellowish, crystalline powder |
|---|---|
| Solubility | Practically insoluble in water, soluble in methanol and ethanol, slightly soluble in chloroform |
| Melting point | 134°C to 136°C |
| pH | 4.0 to 6.0 |
Desloratadine is a long-acting second-generation H1-receptor antagonist which has a selective and peripheral H1-antagonist action. Histamine is a chemical that causes many of the signs that are part of allergic reactions, such as the swelling of tissues. Histamine is released from histamine-storing cells (mast cells) and attaches to other cells that have receptors for histamine. The attachment of the histamine to the receptors causes the cell to be "activated," releasing other chemicals which produce the effects that we associate with allergies. Desloratadine blocks one type of receptor for histamine (the H1 receptor) and thus prevents activation of cells by histamine. Unlike most other antihistamines, Desloratadine does not enter the brain from the blood and, therefore, does not cause drowsiness.
Like other H1-blockers, Desloratadine competes with free histamine for binding at H1-receptors in the GI tract, uterus, large blood vessels, and bronchial smooth muscle. This blocks the action of endogenous histamine, which subsequently leads to temporary relief of the negative symptoms (eg. nasal congestion, watery eyes) brought on by histamine.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Reliable GMP-based manufacturing, strong documentation (DMF/CoA/CTD), timely logistics, and region-specific regulatory support.
Reliable GMP-based manufacturing, strong documentation (DMF/CoA/CTD), timely logistics, and region-specific regulatory support.
Secure HDPE/Fiber drum packaging (usually 25 kg), pharmacopeial grade compliance, stable impurity profile, and controlled residual solvents.
High receptor selectivity with minimal sedation, better potency vs. loratadine, and strong safety for chronic treatment applications.
Store in airtight containers, away from moisture and light, at normal controlled temperatures to maintain stability during transport and warehousing.
Yes — pharmacopeial compliance (BP/USP), local labeling requirements, stability data, and approval status within regional regulatory agencies.
Looking to source Desloratadine or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.