
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
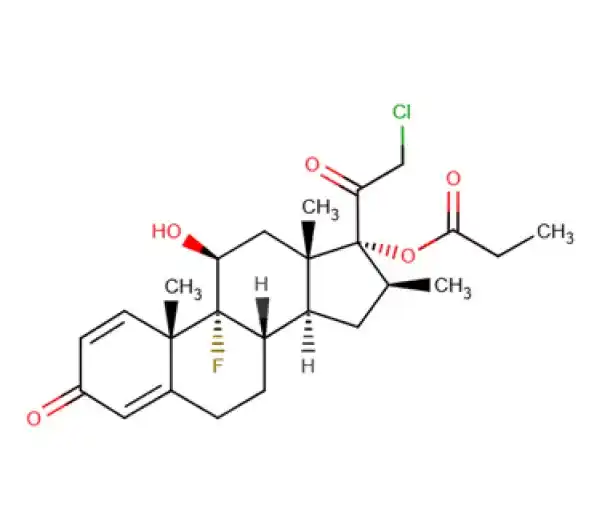
API
USP
C25H32ClFO5
(11β,16β)-21-Chloro-9-fluoro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-diene-17-yl propanoate
25122-46-7
466.97 g/mol
Glucocorticoid
Anti-Inflammatory Agent
| Appearance | White to off-white crystalline powder. |
|---|---|
| Solubility | Practically insoluble in water, freely soluble in alcohol, chloroform, and methylene chloride & slightly soluble in ether |
| Melting point | 196°C to 198°C |
| pH | - |
Corticosteroids bind to the glucocorticoid receptor, inhibiting pro-inflammatory signals, and promoting anti-inflammatory signals. Clobetasol propionate is generally applied twice daily so the duration of action is long. Corticosteroids have a wide therapeutic window as patients may require doses that are multiples of what the body naturally produces. Patients taking corticosteroids should be counselled regarding the risk of hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections.
The short-term effects of corticosteroids are decreased vasodilation and permeability of capillaries, as well as decreased leukocyte migration to sites of inflammation. Corticosteroids binding to the glucocorticoid receptor mediates changes in gene expression that lead to multiple downstream effects over hours to days.
Glucocorticoids inhibit neutrophil apoptosis and demarginating; they inhibit phospholipase A2, which decreases the formation of arachidonic acid derivatives; they inhibit NF-Kappa B and other inflammatory transcription factors; they promote anti-inflammatory genes like interleukin-10.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Global dermatology markets with high treatment incidence of psoriasis, eczema, lichen planus, and other corticosteroid-responsive skin conditions consistently drive demand for this high-potency topical steroid API.
Salius Pharma supplies USP-grade, GMP-certified API with full regulatory support including DMF, CoA, and CTD/eCTD documentation. We offer competitive pricing, flexible export packaging, and reliable logistics across LATAM, MENA, Africa, and Southeast Asia.
Importers should verify DMF acceptance and align usage with local controls for potent steroids. Product must be stored in airtight, light-protected containers to maintain stability and prevent degradation.
It inhibits inflammatory pathways, reduces immune cell activity, and constricts capillaries, which quickly relieves itching, redness, and swelling in severe skin disorders.
Buyers should ensure high purity, tight control of impurities and residual solvents, and particle size suitable for topical formulation in creams, ointments, or lotions, ensuring consistent potency and skin tolerability.
Looking to source Clobetasol Propionate or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.