
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
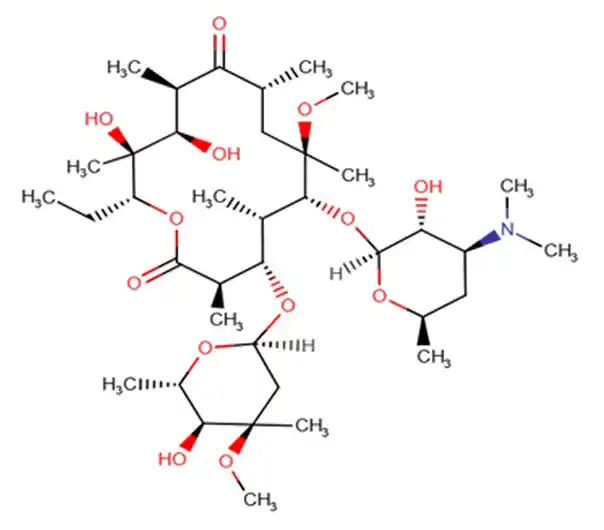
C38H69NO13
(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-{[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy}-14-ethyl-12,13-dihydroxy-4-{[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy}-7-methoxy-3,5,7,9,11,13-hexamethyl-1-oxacyclotetradecane-2,10-dione
81103-11-9
747.9534 g/mol
Macrolide antibiotic
Macrolide antibiotic
| Appearance | White to off-white crystalline powder. |
|---|---|
| Solubility | Freely soluble in: Acetone, methanol, and ethanol. Practically insoluble in: Water |
| Melting point | 220°C to 225°C |
| pH | - |
Clarithromycin is a macrolide antibiotic whose spectrum of activity includes many gram-positive (Staphylococcus aureus, S. pneumoniae, and S. pyogenes) and gram-negative aerobic bacteria (Haemophilus influenzae, H. parainfluenzae, and Moraxella catarrhalis), many anaerobic bacteria, some mycobacteria, and some other organisms including Mycoplasma, Ureaplasma, Chlamydia, Toxoplasma, and Borrelia. Other aerobic bacteria that clarithromycin has activity against include C. pneumoniae and M. pneumoniae. Clarithromycin has an in-vitro activity that is similar or greater than that of erythromycin against erythromycin-susceptible organisms. Clarithromycin is usually bacteriostatic but may be bactericidal depending on the organism and the drug concentration.
Clarithromycin is first metabolized to 14-OH clarithromycin, which is active and works synergistically with its parent compound. Like other macrolides, it then penetrates bacteria cell wall and reversibly binds to domain V of the 23S ribosomal RNA of the 50S subunit of the bacterial ribosome, blocking translocation of aminoacyl transfer-RNA and polypeptide synthesis. Clarithromycin also inhibits the hepatic microsomal CYP3A4 isoenzyme and P-glycoprotein, an energy-dependent drug efflux pump.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Primary use: Respiratory tract infections—such as community-acquired pneumonia, bronchitis, sinusitis, and pharyngitis—remain the largest application segment globally, including Latin America.
Other key areas: Skin and soft tissue infections (e.g., cellulitis, impetigo); Helicobacter pylori eradication in ulcer therapy as part of combination regimens; and prophylaxis/treatment of infections like Mycobacterium avium complex (MAC), particularly in immunocompromised patients.
Salius Pharma tailor’s regulatory dossiers (DMFs, CoAs, CTD/eCTD) to meet authorities such as COFEPRIS (Mexico), INVIMA (Colombia), DIGEMID (Peru), and ISP (Chile).
Antibiotic resistance: Extended or repeated use promotes resistance development and superinfections, including Clostridioides difficile colitis—which may appear up to two months post therapy.
QT prolongation and cardiac risks: Prolonged use increases risk of arrhythmias such as Torsades de Pointes, especially in patients with electrolyte imbalances (e.g. hypokalemia, hypomagnesemia), underlying heart disease, or concurrent QT prolonging drugs.
Hepatotoxicity: There is a low but significant risk of acute liver injury (including cholestatic hepatitis), which may be severe or fatal.
Clarithromycin is a macrolide antibiotic that binds reversibly to the 50S ribosomal subunit, inhibiting the peptidyl transferase step and impairing protein synthesis. It is generally bacteriostatic, although it can be bactericidal against certain pathogens at higher concentrations. It is actively concentrated in phagocytic cells, enhancing delivery to infection sites.
Common (≥ 3–10%): Gastrointestinal symptoms such as nausea, diarrhea, abdominal pain, and altered taste (often metallic or bitter).
Less common: Headache, dizziness, rash, insomnia.
Looking to source Clarithromycin or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.