
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
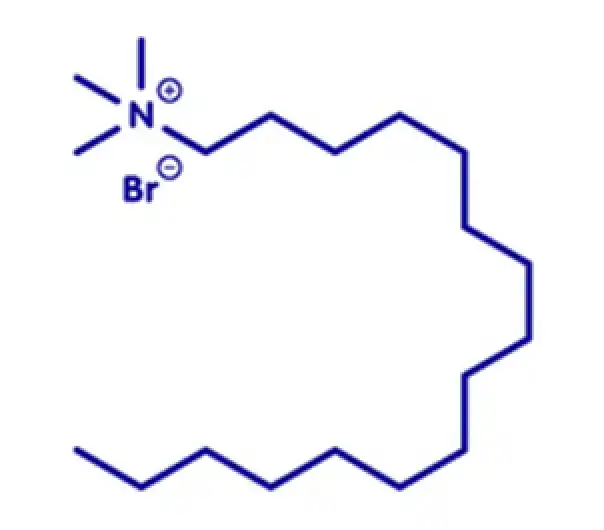
API
BP/USP
C17H38BrN
hexadecyl(trimethyl)azanium bromide
8044-71-1
336.4 g/mol
Quaternary ammonium salts
Anti-Infective Agents
| Appearance | White powder. |
|---|---|
| Solubility | Freely soluble in water and ethanol, but practically insoluble in ether |
| Melting point | 237 to 243 °C |
| pH | 4.0 to 5.0. |
Cetrimide, is a topical antiseptic and surfactant used for cleaning wounds, treating minor skin issues, and preventing infection. It's a quaternary ammonium compound, acting as a detergent and disinfectant to eliminate bacteria and fungi. While generally safe, some individuals may experience skin irritation or allergic reactions.
Cetrimide is a quaternary ammonium antiseptic with bactericidal action against gram positive and some gram negative (at higher concentrations) organisms, but ineffective against bacterial spore. It has variable antifungal activity and effective against some viruses.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Cetrimide is commonly used in dermatological and surgical settings as an antiseptic and disinfectant. It is prescribed for wound cleansing, minor cuts and abrasions, pre-operative skin disinfection, and in the treatment of skin infections caused by bacteria. It is also used in topical formulations and first-aid antiseptic products.
Salius Pharma ensures smooth export of Cetrimide by providing complete regulatory documentation including DMFs, Certificates of Analysis (CoAs), and CTD/eCTD dossiers, customized for authorities such as ANMAT (Argentina), DIGEMID (Peru). This helps international buyers comply with import regulations and streamline market entry.
Cetrimide API is packed in export-grade HDPE drums with moisture-proof inner liners, sealed to prevent contamination, leakage, or degradation. Packaging is designed to withstand long-distance transport via sea or air freight, ensuring the API maintains its potency and stability upon arrival.
We coordinate temperature-controlled shipments, robust palletization, and secure handling procedures to ensure compliance with international freight norms. Our logistics team monitors the entire supply chain, from warehouse dispatch to customs clearance, for timely and safe delivery.
Each shipment includes GMP certificates, batch-specific CoAs, MSDS, stability data, and shipping compliance forms. Additional export-ready documents like harmonized tariff codes (HS), product traceability, and regulatory declarations are provided to meet customs and local authority requirements.
Yes — our export team offers guidance on customized labeling, regulatory filings, and documentation to meet local authority requirements, ensuring smooth clearance in countries like Argentina, Colombia, and Peru.
Looking to source Cetrimide or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.