
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
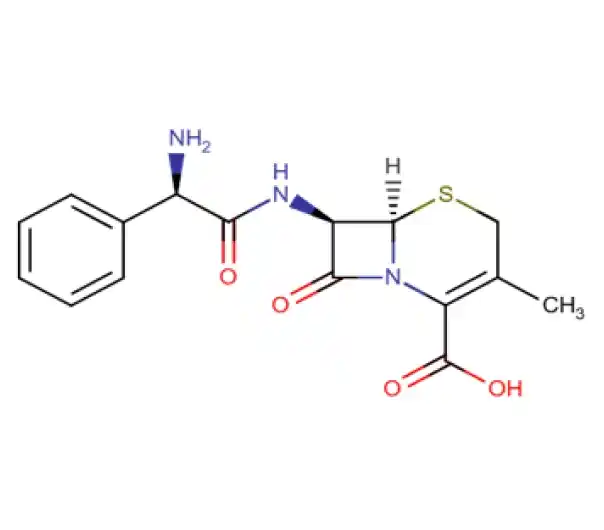
C16H17N3O4S
(6R,7R)-7-[(2R)-2-amino-2-phenylacetamido]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
15686-71-2
347.389 g/mol
Cephalosporins
Antibiotic- Cephalosporins
| Appearance | White to off-white crystalline powder. |
|---|---|
| Solubility | Freely soluble in water; practically insoluble in alcohol, chloroform, and ether. |
| Melting point | 325–328 °C |
| pH | 4.0 to 6.0. |
Cephalexin is the first of the first generation cephalosporins. This antibiotic contains beta lactam and dihydrothiazide. Cephalexin is used to treat several susceptible bacterial infections through inhibition of cell wall synthesis. Cephalexin was approved by the FDA on 4 January 1971.
Cephalexin is a first-generation cephalosporin antibiotic. Cephalosporins contain a beta lactam and dihydrothiazide. Unlike penicillins, cephalosporins are more resistant to the action of beta lactamase. Cephalexin inhibits bacterial cell wall synthesis, leading breakdown and eventually cell death.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Salius Pharma manufactures Cephalexin API according to BP/USP/EP standards and provides comprehensive documentation including GMP certificates, Certificates of Analysis (CoA), stability data, and DMFs, ensuring compliance for regulatory submissions worldwide.
Cephalexin API is packed in HDPE drums with tamper-evident, moisture-resistant liners, designed to maintain stability during long sea or air freight shipments. Packaging is compliant with international norms to prevent contamination and degradation.
Our logistics team coordinates secure palletization, temperature-controlled shipping (if required), and real-time tracking, ensuring timely delivery while maintaining product integrity during transit to international destinations.
Shipments come with batch-specific CoAs, MSDS, GMP certifications, stability data, and customs compliance forms. Additional documents such as DMF letters, FSC, or CTD/eCTD dossiers can be provided depending on the importer’s regulatory requirements.
Yes — we offer guidance on country-specific regulatory filings, labeling requirements, and documentation to streamline customs clearance and market approval for international buyers.
We maintain strategic inventory and scalable production capacity to meet large-volume demands while ensuring uniform quality and on-time delivery for global pharmaceutical manufacturers.
Looking to source Cephalexin or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.