
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
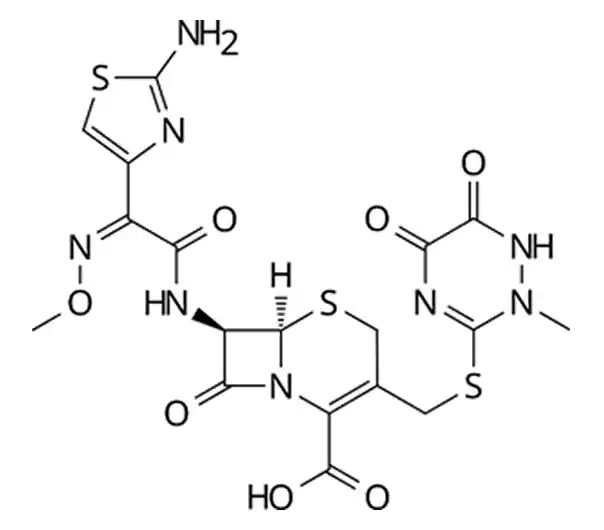
C₁₈H₁₆N₈Na₂O₇S₃•3.5H₂O
tetrasodium (6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino]-3-[(2-methyl-5,6-dioxo-1H-1,2,4-triazin-3-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate;heptahydrate
104376-79-6
598.54 g/mol
Cephalosporins
Antibiotic- Cephalosporins
| Appearance | White to yellowish crystalline powder |
|---|---|
| Solubility | Freely soluble in water, sparingly soluble in methanol, insoluble in acetone and chloroform |
| Melting point | >200 °C |
| pH | 6.0 to 8.0 |
Ceftriaxone Sodium is a broad-spectrum third-generation cephalosporin antibiotic. It has a very long half-life compared to other cephalosporins and is high penetrable into the meninges, eyes, and inner ear. Ceftriaxone has broader and stronger gram-negative coverage than first or second-generation cephalosporins, but worse activity against methicillin-susceptible S.aureus.
Ceftriaxone works by inhibiting the mucopeptide synthesis in the bacterial cell wall.10,11 The beta-lactam moiety of ceftriaxone binds to carboxypeptidases, endopeptidases, and transpeptidases in the bacterial cytoplasmic membrane. These enzymes are involved in cell-wall synthesis and cell division. Binding of ceftriaxone to these enzymes causes the enzyme to lose activity; therefore, the bacteria produce defective cell walls, causing cell death.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
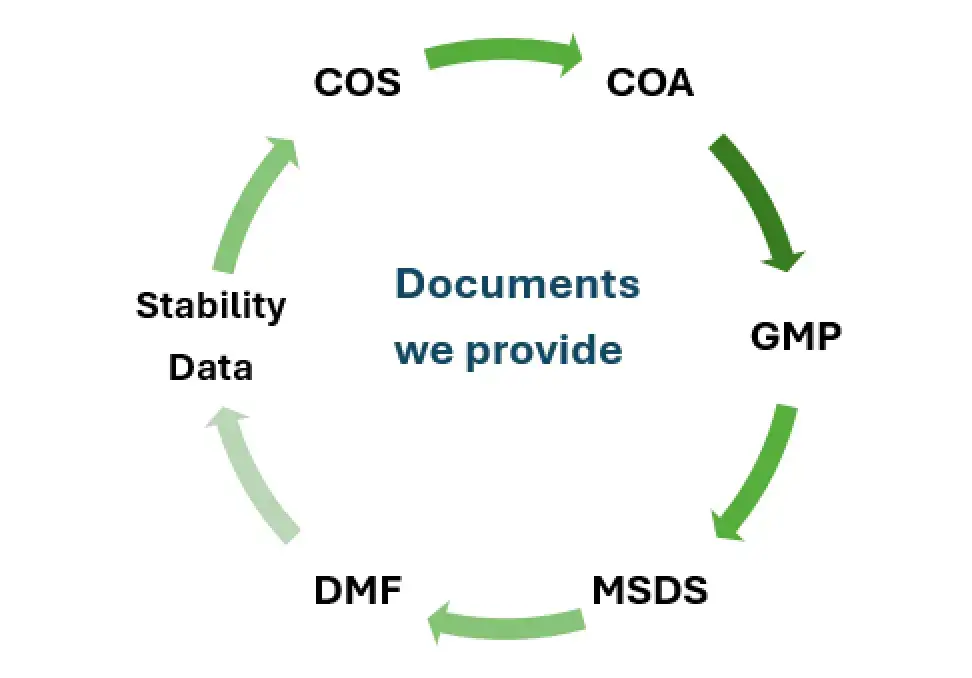
Salius Pharma manufactures Ceftriaxone Sodium API in compliance with BP/USP/EP standards and provides full documentation packages including GMP certification, Certificates of Analysis (CoA), DMFs, and stability data, supporting smooth registration in global markets.
The API is packed in high-strength HDPE drums with moisture-proof inner liners to maintain product integrity during long-distance sea or air transport. Packaging is designed to prevent contamination, leakage, and degradation.
For global markets, we provide customized regulatory dossiers, including CTD/eCTD submissions, CoAs, MSDS, and GMP certificates, tailored to meet the requirements of importing authorities in countries like Nigeria, Colombia, Vietnam, and Egypt.
Salius Pharma coordinates temperature-controlled shipping (if required), palletization, and secure handling to protect the API during transit. Our logistics team ensures on-time delivery while maintaining compliance with international freight regulations.
Through strategic inventory management, scalable production capacity, and robust quality control, Salius Pharma ensures consistent API availability for global manufacturers, minimizing the risk of supply interruptions.
Looking to source Ceftriaxone Sodium or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.