
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
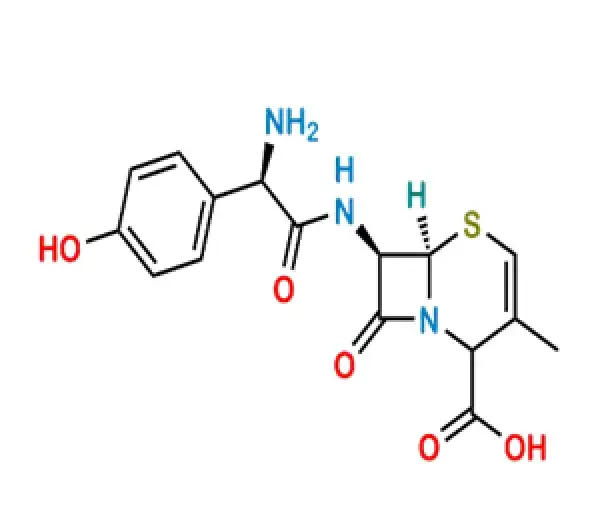
C16H17N3O5S
(6R,7R)-7-[(2R)-2-amino-2-(4-hydroxyphenyl)acetamido]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
50370-12-2
363.388 g/mol
Carbonylimidazoles
Antibiotic- First-Generation Cephalosporins
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in water, Slightly soluble in methanol and ethanol, Practically insoluble in chloroform and ether |
| Melting point | 208–220 °C |
| pH | 4.0 to 6.0 |
Cefadroxil is a cephalosporin antibiotic used in the treatment of various bacterial infections, such as urinary tract infections, skin and skin structure infections, and tonsillitis.
Like all beta-lactam antibiotics, cefadroxil binds to specific penicillin-binding proteins (PBPs) located inside the bacterial cell wall, causing the inhibition of the third and last stage of bacterial cell wall synthesis. Cell lysis is then mediated by bacterial cell wall autolytic enzymes such as autolysins; it is possible that cefadroxil interferes with an autolysin inhibitor.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Looking to source Cefadroxil or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.