
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP/EP
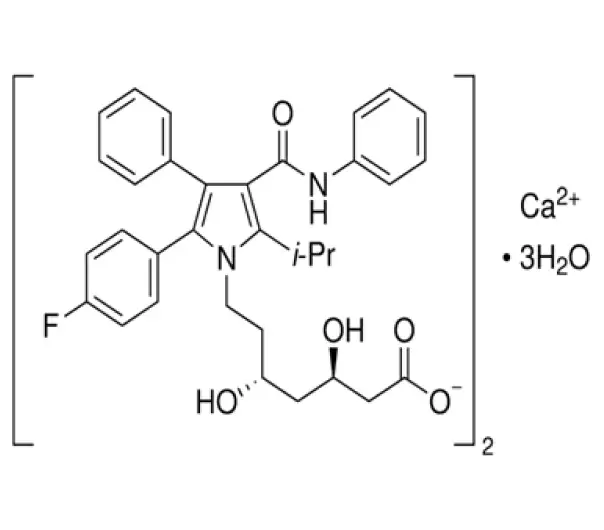
C66H68CaF2N4O10
calcium;(3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoate;trihydrate
134523-03-5
1155.342 g/mol
Diphenylpyrroles
Hypolipidemic Agent
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; freely soluble in methanol, ethanol, and acetonitrile |
| Melting point | 159–160°C |
| pH | 7.0–9.0 (in aqueous suspension or solution form) |
Atorvastatin is a lipid-lowering drug included in the statin class of medications. By inhibiting the endogenous production of cholesterol in the liver, statins lower abnormal cholesterol and lipid levels, and ultimately reduce the risk of cardiovascular disease. Atorvastatin is indicated, in combination with dietary modifications, to prevent cardiovascular events in patients with cardiac risk factors and/or abnormal lipid profiles.
Atorvastatin is a statin medication and a competitive inhibitor of the enzyme HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) reductase, which catalyzes the conversion of HMG-CoA to mevalonate, an early rate-limiting step in cholesterol biosynthesis.1,8 Atorvastatin acts primarily in the liver, where decreased hepatic cholesterol concentrations stimulate the upregulation of hepatic low-density lipoprotein (LDL) receptors, which increases hepatic uptake of LDL.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Looking to source Atorvastatin Calcium or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.