
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
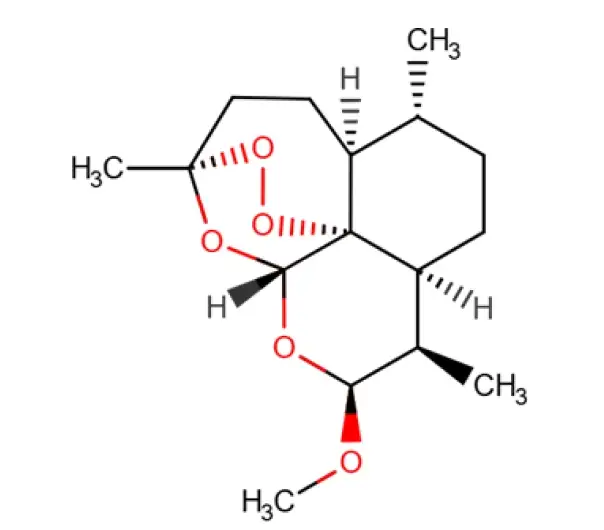
C16H26O5
(1R,4S,5R,8S,9R,10S,12R,13R)-10-methoxy-1,5,9-trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.0^{4,13}.0^{8,13}]hexadecane
71963-77-4
298.3746 g/mol
Artemisinins
Antimalarials
| Appearance | White to slightly yellow crystalline powder |
|---|---|
| Solubility | Practically insoluble in water, freely soluble in organic solvents like chloroform, ethanol, and acetone |
| Melting point | 86–88 °C |
| pH | - |
Artemether is an antimalarial agent used in combination with lumefantrine for the treatment of acute uncomplicated malaria caused by Plasmodium falciparum. In the body, artemether is metabolized into the active metabolite dihydroartemisinin. The drug works against the erythrocytic stages of P. falciparum by inhibiting nucleic acid and protein synthesis. Artemether is administered in combination with lumefantrine for improved efficacy.
In the body, artemether is metabolized into the active metabolite dihydroartemisinin. The drug works against the erythrocytic stages of P. falciparum by inhibiting nucleic acid and protein synthesis. Artemether is administered in combination with lumefantrine for improved efficacy. The generally accepted mechanism of action of peroxide antimalarials involves interaction of the peroxide-containing drug with heme, a hemoglobin degradation byproduct, derived from proteolysis of hemoglobin.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Looking to source Artemether or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.