
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
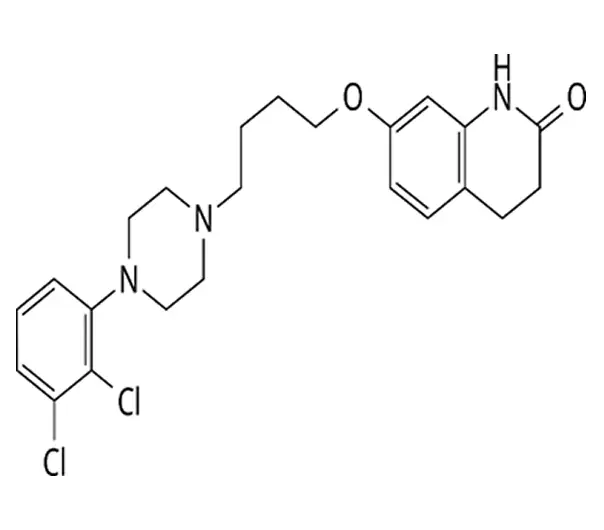
C23H27Cl2N3O2
7-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one
129722-12-9
448.385 g/mol
Phenylpiperazines
Antipsychotic Agents
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Very slightly soluble in water, soluble in methanol and ethanol, freely soluble in chloroform, practically insoluble in n-hexane |
| Melting point | 139–141 °C |
| pH | - |
Aripiprazole is an atypical antipsychotic used in the treatment of a wide variety of mood and psychotic disorders, such as schizophrenia, bipolar I, major depressive disorder, irritability associated with autism, and Tourette's syndrome. It is also indicated as an injection for agitation associated with schizophrenia or bipolar mania.
The antipsychotic action of aripiprazole is likely due to its partial agonist activity on D2 and 5-HT1A receptors as well as its antagonist activity at 5-HT2A receptors; however, the exact mechanism has not been fully elucidated. One of the mechanisms that have been proposed is that aripiprazole both stimulates and inhibits dopamine as it engages the D2 receptor. It lowers dopamine neuronal firing at high dopamine concentrations and increases dopamine firing at low concentrations.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Looking to source Aripiprazole or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.