
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
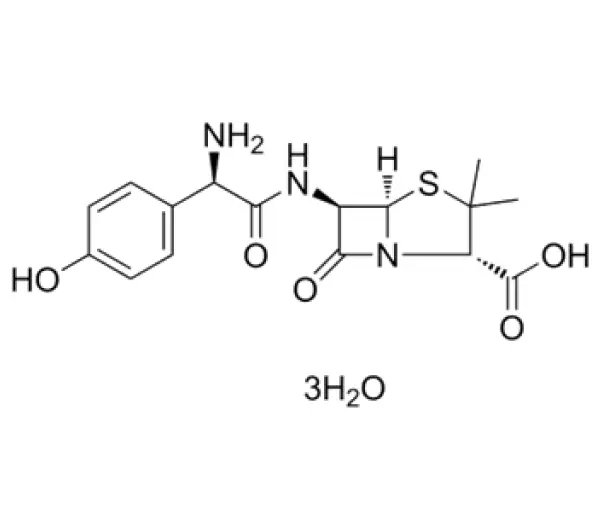
C16H19N3O5S · 3H2O
(2S,5R,6R)-6-[[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid trihydrate
61336-70-7
419.45 g/mol
Penicillins
Antibiotics
| Appearance | White or almost white crystalline powder |
|---|---|
| Solubility | Freely soluble in water, slightly soluble in alcohol and other organic solvents |
| Melting point | 194–200 °C |
| pH | 4.0 to 5.5 |
Amoxicillin is a penicillin derivative used for the treatment of infections caused by gram-positive bacteria, in particular streptococcal bacteria causing upper respiratory tract infections. Amoxicillin competitively inhibits penicillin binding proteins, leading to upregulation of autolytic enzymes and inhibition of cell wall synthesis. Amoxicillin has a long duration of action as it is usually given twice daily.
Amoxicillin competitively inhibits penicillin-binding protein 1 and other high molecular weight penicillin binding proteins. Penicillin bind proteins are responsible for glycosyltransferase and transpeptidase reactions that lead to cross-linking of D-alanine and D-aspartic acid in bacterial cell walls.10 Without the action of penicillin binding proteins, bacteria upregulate autolytic enzymes and are unable to build and repair the cell wall, leading to bacteriocidal action.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Looking to source Amoxicillin Trihydrate or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.