
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
IP/BP/USP/EP/JP
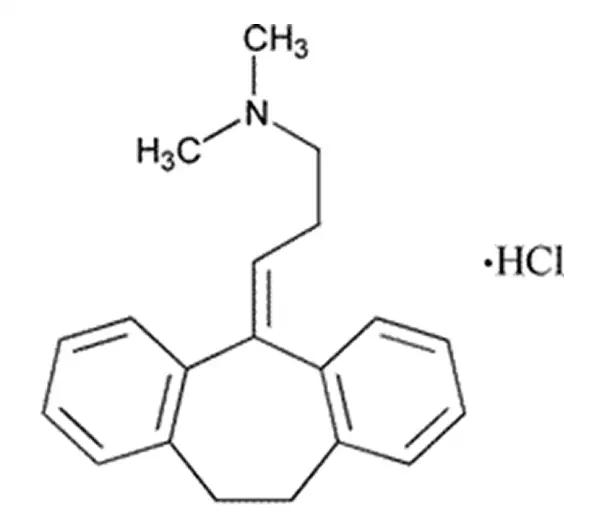
C20H24ClN
dimethyl(3-{tricyclo[9.4.0.0^{3,8}]pentadeca-1(15),3,5,7,11,13-hexaen-2-ylidene}propyl)amine hydrochloride
549-18-8
313.864g/mol
Dibenzocycloheptenes
Antidepressive Agents
| Appearance | White or almost white crystalline powder |
|---|---|
| Solubility | Freely soluble in water, ethanol, and methanol; slightly soluble in chloroform |
| Melting point | 195–199 °C |
| pH | 5.0–6.0 |
Amitriptyline is a tricyclic antidepressant that has been used to treat depression for decades. ELAVIL, a previously approved branded product of amitriptyline, was first approved by the FDA in 1961. Amitriptyline has been investigated in the treatment of pain-related conditions, attributed to its analgesic properties.
It is suggested that amitriptyline inhibits the membrane pump mechanism responsible for the re-uptake of transmitter amines, such as norepinephrine and serotonin, thereby increasing their concentration at the synaptic clefts of the brain. These amines are important in regulating mood. The monoamine hypothesis in depression, one of the oldest hypotheses, postulates that deficiencies of serotonin (5-HT) and/or norepinephrine (NE) neurotransmission in the brain lead to depressive effects. This drug counteracts these mechanisms, and this may be the mechanism of amitriptyline in improving depressive symptoms.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Looking to source Amitriptyline Hydrochloride or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.