
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
USP/EP
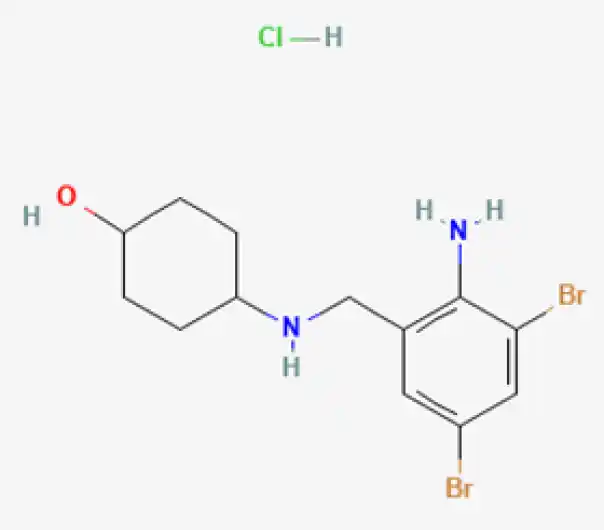
C13H18Br2N2O
(1r,4r)-4-{[(2-amino-3,5-dibromophenyl)methyl]amino}cyclohexan-1-ol
18683-91-5
378.108 g/mol
Phenylmethylamines
Mucolytic agent
| Appearance | White powder |
|---|---|
| Solubility | soluble in organic solvents such as ethanol, methanol, DMSO, and dimethyl formamide (DMF) |
| Melting point | 235 °C |
| pH | 7.7 |
Ambroxol hydrochloride is a secretolytic agent used in the treatment of respiratory diseases associated with viscid or excessive mucus. It is the active ingredient of Mucosolvan, Lasolvan or Mucoangin. The substance is a mucoactive drug with several properties including secretolytic and secretomotoric actions that restore the physiological clearance mechanisms of the respiratory tract which play an important role in the body’s natural defense mechanisms.
Ambroxol hydrochloride is a basic inorganic salt that acts by neutralizing hydrochloric acid in gastric secretions. Ambroxol hydrochloride is slowly solubilized in the stomach and reacts with hydrochloric acid to form aluminum chloride and water. It also inhibits the action of pepsin by increasing the pH and via adsorption. Cytoprotective effects may occur through increases in bicarbonate ion (HCO3-) and prostaglandins.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO-GMP, ISO 9001:2015, FDA-audited facilities |
| Affordability & Generic Access | Cost-competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Salius Pharma supplies Ambroxol Hydrochloride API in export-grade HDPE drums with protective inner liners to prevent moisture, contamination, or degradation during transit, ensuring the API reaches formulators in optimal condition.
Our API shipments include Certificate of Analysis (CoA), MSDS, GMP certification, stability data, and batch-specific quality reports. Additional documentation like DMF or CEP can also be provided upon request to meet importing country regulations.
Through strict in-process controls, validated manufacturing processes, and comprehensive analytical testing, Salius Pharma guarantees consistent purity, potency, and impurity profile in every batch of Ambroxol Hydrochloride API.
Yes — Salius Pharma can provide custom export labels, product traceability details, handling instructions, and documentation formatted to buyer requirements, ensuring smooth compliance with international customs and regulatory authorities.
We maintain strategic inventory, flexible production scheduling, and robust logistics planning to support large-scale orders, ensuring timely delivery and uninterrupted supply for global pharmaceutical manufacturers.
Looking to source Ambroxol Hydrochloride or other high-quality pharmaceutical products? We’re here to help. Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support, our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.